FiberLocker® System
Mechanical Augmentation of Rotator Cuff Repair through Fiberlocking
Fiberlock™
fi·ber·lock | \ˈfaɪbɚˌlɑk\ verb
To reinforce soft tissue, typically tendon, by interweaving patch fibers into tissue, resulting in immediate mechanical strength to the repair construct.
The FiberLocker® System is commercially available in the U.S.

FiberLocker® Instrument
The single-use surgical instrument comprises a reciprocating microneedle at its tip, running at 2,500 rpm, which facilitates the attachment of the patch.
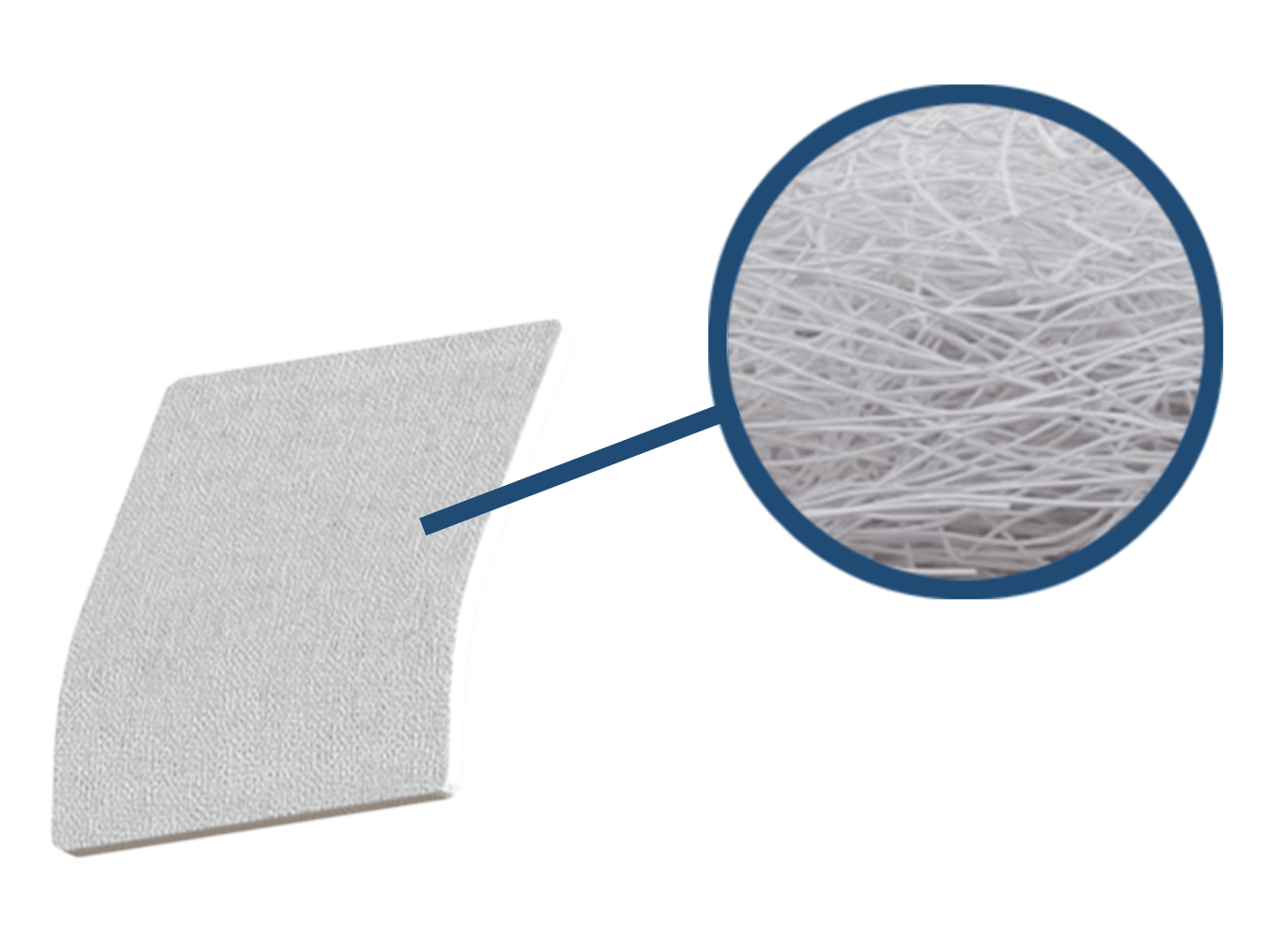
SpeedPatch®
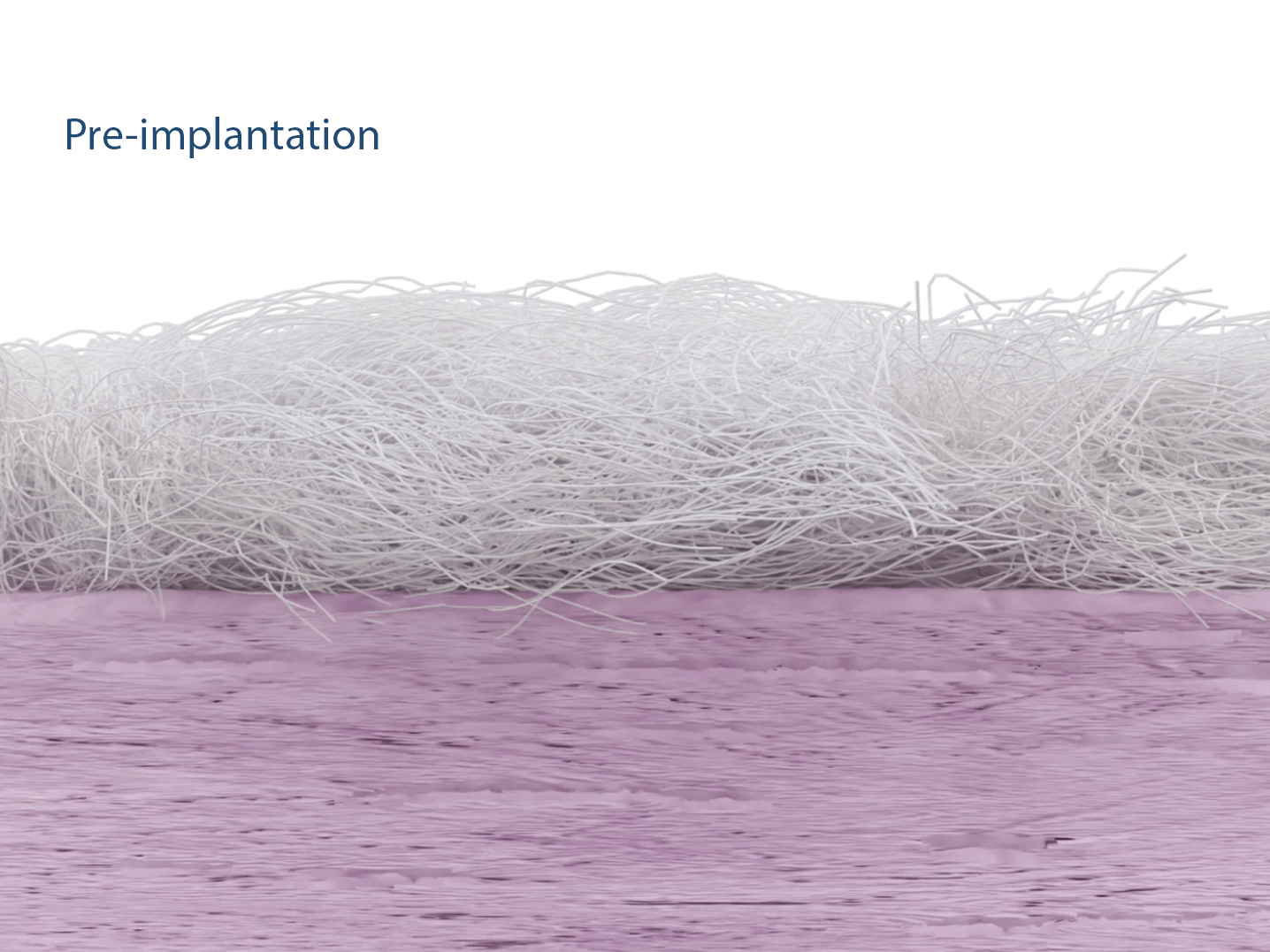
The implant is made of non-woven polyester (Polyethylene terephthalate) fibers, forming a mechanically durable and porous scaffold. The patch measures 30 × 25 mm in size.
Ease of Use
The simple implantation is completed in just 90 seconds.1
Immediate Mechanical Strength
Ex vivo testing has shown a significantly increased biomechanical strength of augmented rotator cuff repairs at time zero.2,3
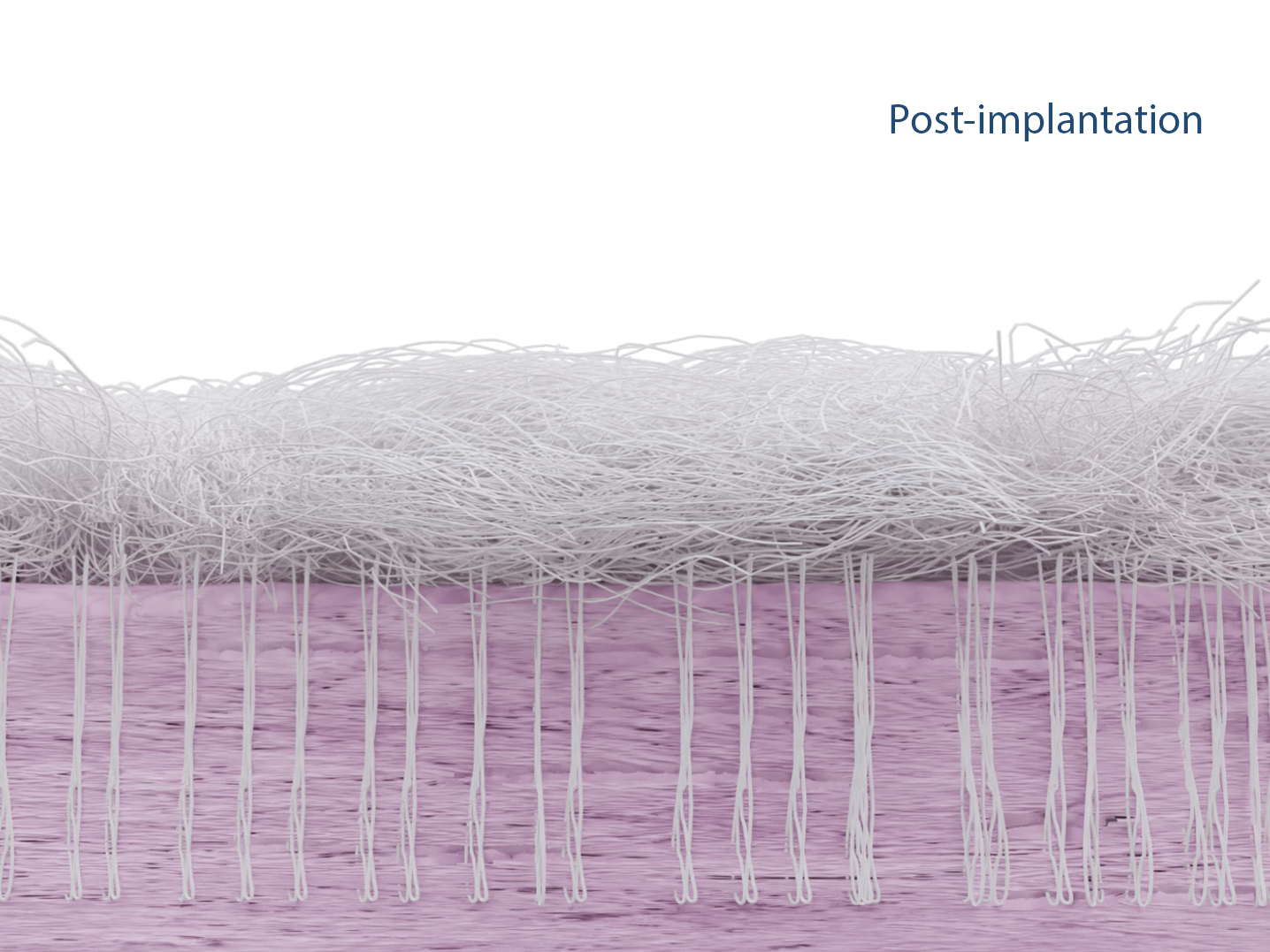
Cellular Ingrowth
Animal studies demonstrated cell infiltration into the porous scaffold.2,4
Clinical Application
Clinical Challenge
Rotator cuff tears affect between 20.4% and 34% of the general population.5-7 Despite surgical repair, retear rates remain high—ranging from 24% to 94%.8,9 Suture cut-through, where sutures pull through the tendon tissue, is the primary cause of failure, accounting for 86% of all cases.10 This highlights the compromised mechanical integrity of the repaired tendon. Until now, no augmentation system has provided sufficient mechanical reinforcement to limit tendon retears and support healing effectively.
Technology
The patented Fiberlock™ technology enables the formation of a strong, interwoven network between the patch and tendon tissue. During implantation, the instrument’s reciprocating needle pushes implant fibers into the underlying soft tissue, securing the patch across the entire patch–tendon interface. This results in immediate mechanical augmentation.2,3
Clinical Application
Clinical Challenge
Rotator cuff tears affect between 20.4% and 34% of the general population.5-7 Despite surgical repair, retear rates remain high—ranging from 24% to 94%.8,9 Suture cut-through, where sutures pull through the tendon tissue, is the primary cause of failure, accounting for 86% of all cases.10 This highlights the compromised mechanical integrity of the repaired tendon. Until now, no augmentation system has provided sufficient mechanical reinforcement to limit tendon retears and support healing effectively.
Technology
The patented Surgical-Fiberlock® technology enables the formation of a strong, interwoven network between the patch and tendon tissue. During implantation, the instrument’s reciprocating needle pushes implant fibers into the underlying soft tissue, securing the patch across the entire patch–tendon interface. This results in immediate mechanical augmentation.2,3
Surgical Procedure
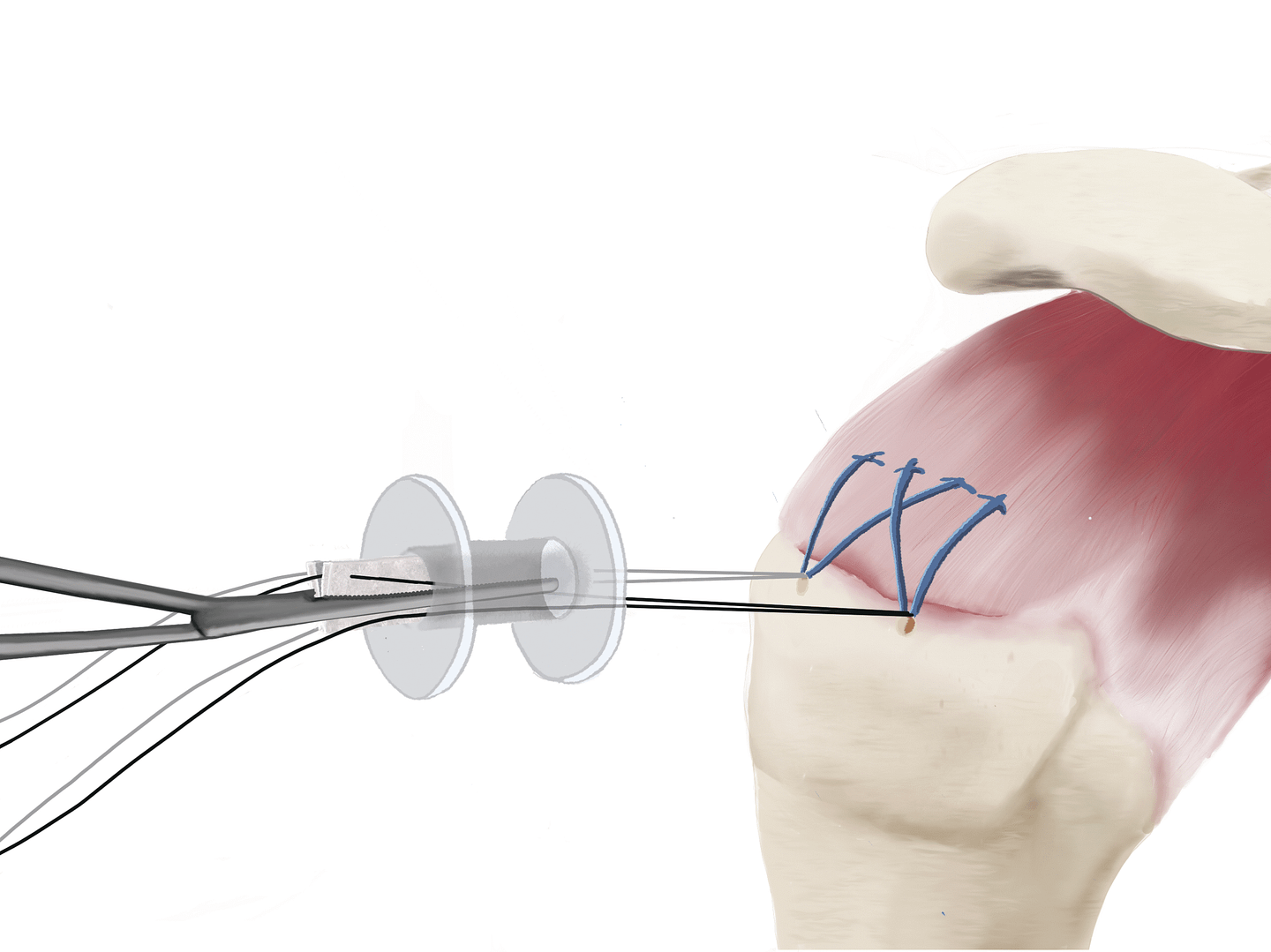
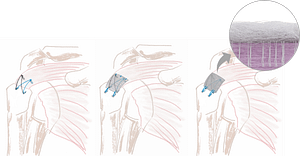
1. Delivery
During or after the repair of the rotator cuff tendon, the SpeedPatch® can be easily delivered according to each surgeon’s preference.
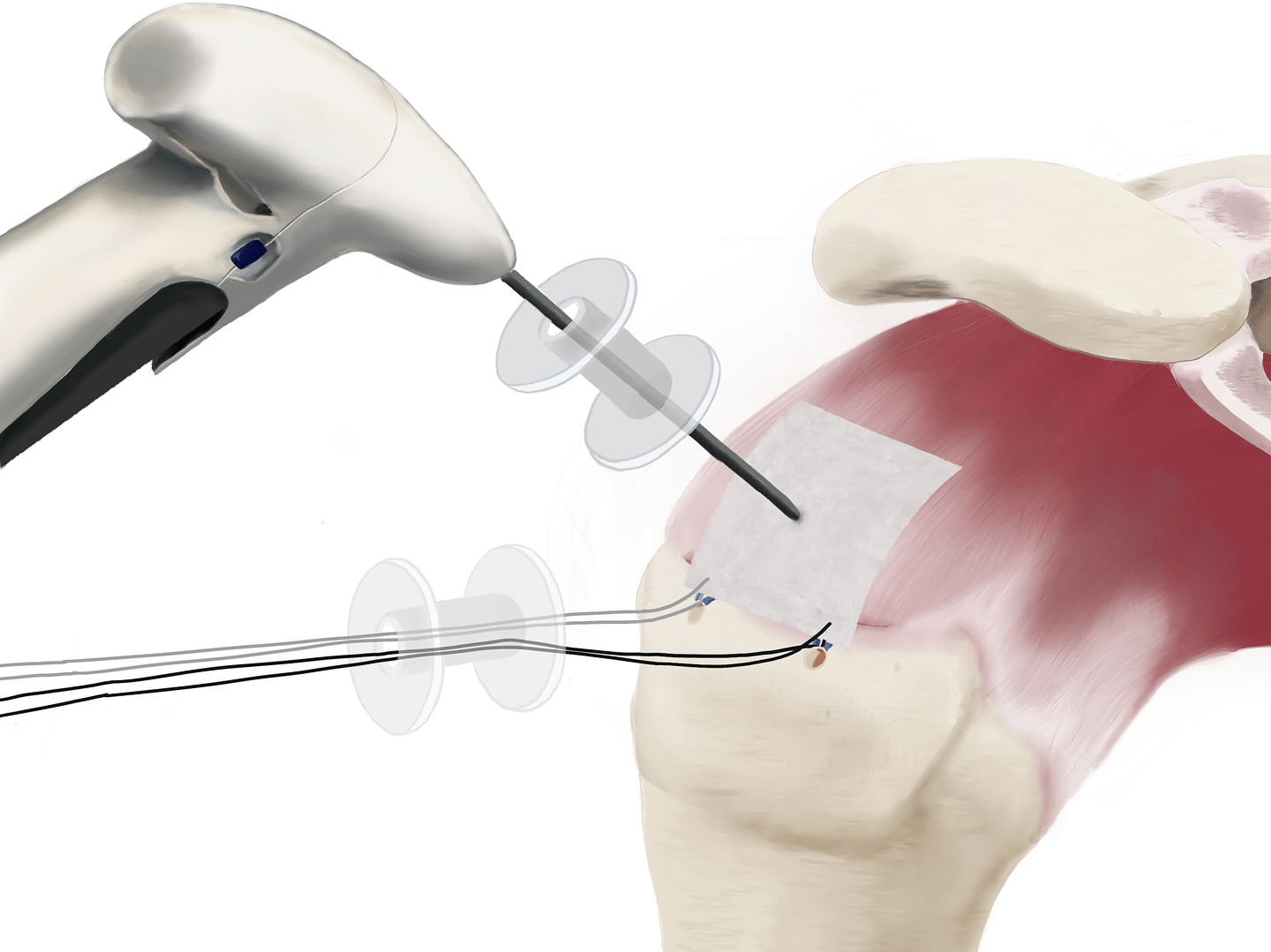
2. Implantation
The SpeedPatch® is secured to the tendon tissue using the FiberLocker® Instrument. In just 90 seconds, the felting technology enables a secure implantation across the entire patch-tendon interface.
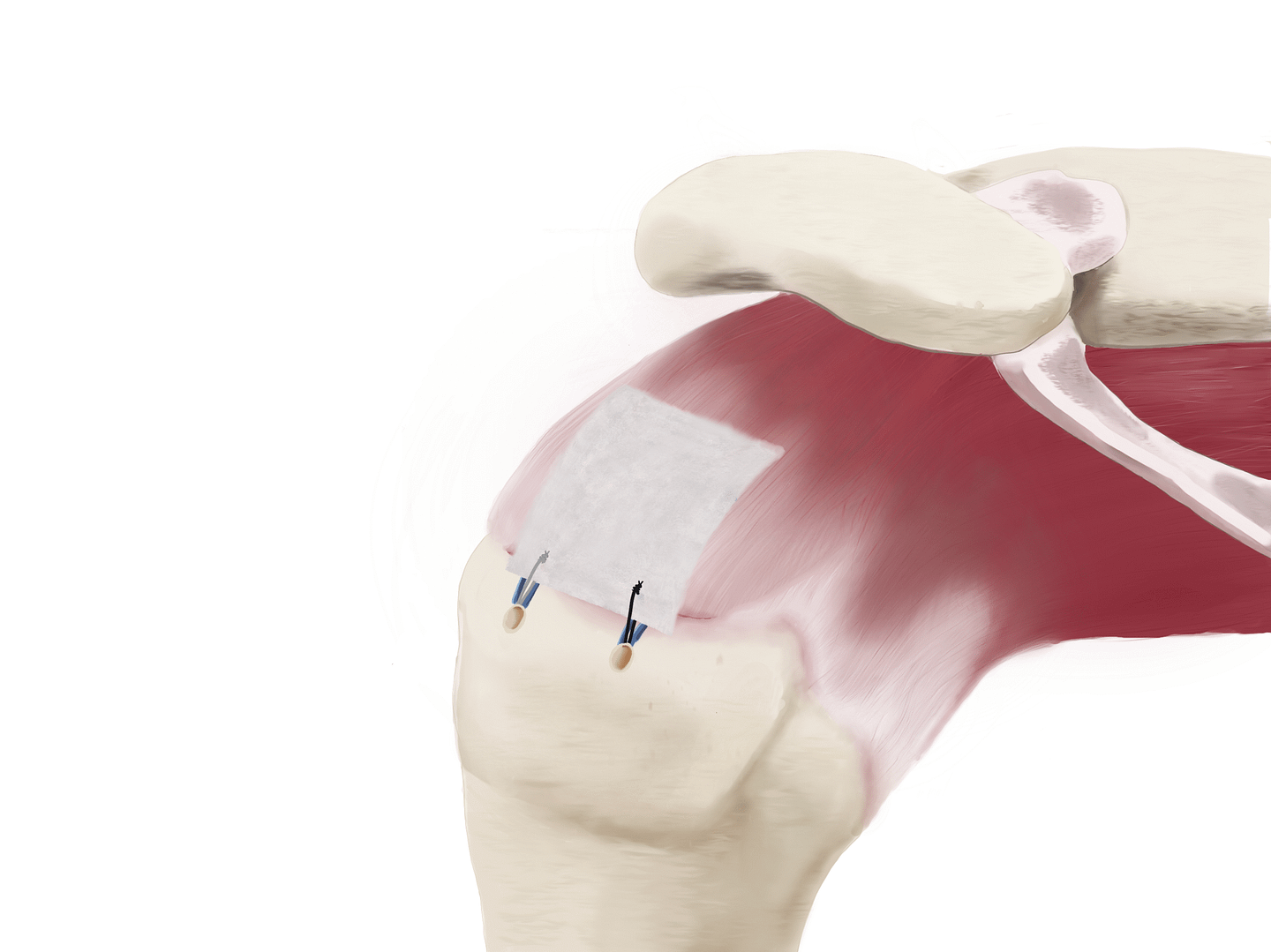
3. Lateral Attachment
Lateral patch-to-bone fixation using sutures and anchors completes the construct.
This technique is only an example. Variations according to each surgeon‘s preference can be applied. Please review the IFU and surgical technique for complete guidance.
The FiberLocker® System is currently only commercially available in the U.S.
Surgical Procedure

1. Delivery
During or after the repair of the rotator cuff tendon, the SpeedPatch® can be easily delivered according to each surgeon’s preference.

2. Implantation
The SpeedPatch® is secured to the tendon tissue using the FiberLocker® Instrument. In just 90 seconds, the felting technology enables a secure implantation across the entire patch-tendon interface.

3. Lateral Attachment
Lateral patch-to-bone fixation using sutures and anchors completes the construct.
This technique is only an example. Variations according to each surgeon‘s preference can be applied. Please review the IFU and surgical technique for complete guidance.
The FiberLocker® System is currently only commercially available in the U.S.
Scientific Evidence
Biomechanical Data
Biological Data
Clinical Data
Currently, two prospective clinical studies investigating the performance and safety of the FiberLocker® System are ongoing.
Sources
Scientific Evidence
Blank
Biomechanical Data
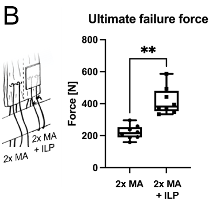
A peer-reviewed ex vivo ovine study (n = 8) revealed significantly increased ultimate tensile strength (UTS) at time zero when augmenting a double Mason Allen stitch with the FiberLocker® System (417 ± 86 N) compared to a non-augmented control group (221 ± 43 N). ²
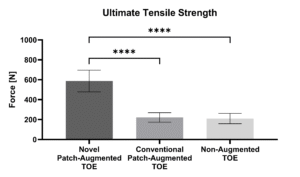
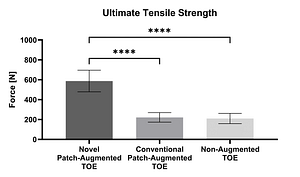
In a peer-reviewed ex vivo ovine study (n = 18), the mechanical strength of augmented and non-augmented Transosseous Equivalent (TOE) repairs was compared. Detached infraspinatus tendons were repaired to the humeri using anchors and sutures. Thereby, the FiberLocker® System-augmented TOE (587.3 ± 108.6 N) demonstrated significantly higher UTS at time zero compared to both Pitch-Patch augmented TOE (221.7 ± 48.1 N) and the non-augmented TOE (210.5 ± 51.5 N). ³
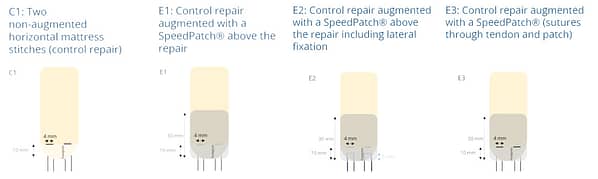
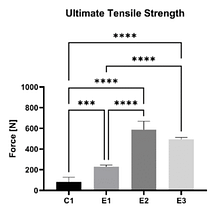
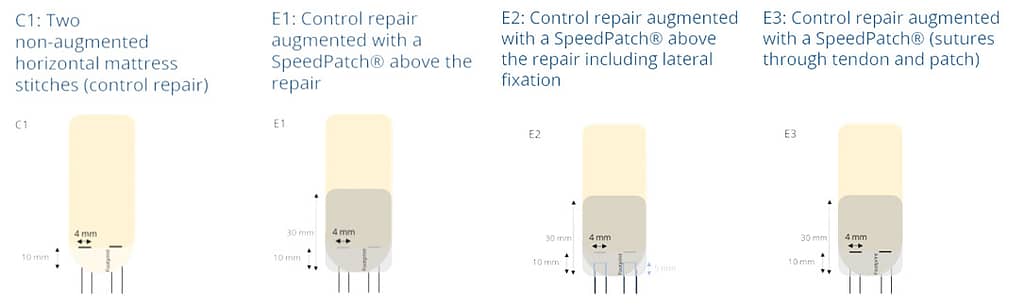
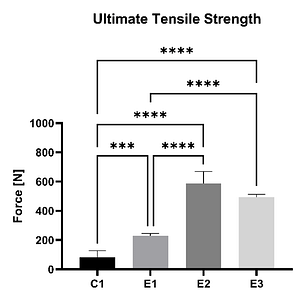
In an ex vivo ovine study (n = 20), the mechanical strength of a non-augmented horizontal mattress suture configuration (C1) was compared to three different SpeedPatch®–augmented suture configurations (E1-E3). Thereby, all augmented constructs demonstrated significantly increased UTS compared to the control at time zero, irrespective of the suture-patch configuration.¹¹
Biological Data
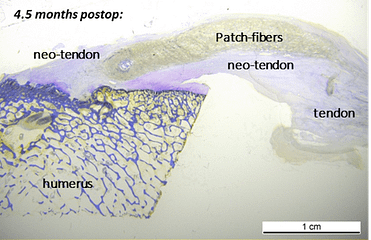
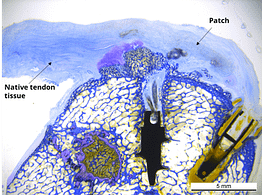
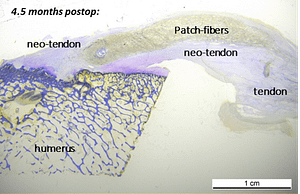
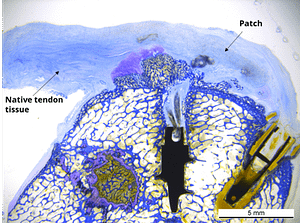
In a published ovine in vivo study, the biological response to the SpeedPatch® was evaluated after 6 and 13 weeks of implantation (n = 8). The results demonstrated longitudinally aligned tissue growth, excellent patch integration into the host tissue, and no adverse tissue reactions.²
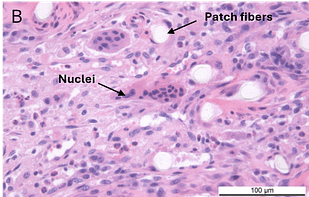
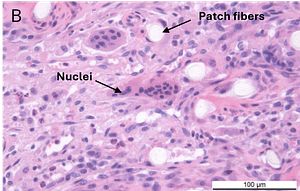
In a second GLP-compliant in vivo ovine study (n = 12), the biological performance of the SpeedPatch® was evaluated at 6 and 12 weeks post-implantation. No adverse local or systemic side effects were observed. The results revealed cell ingrowth into the porous scaffold, a smooth transition between the patch and tendon tissue, and directional collagen alignment above the patch. Furthermore, secure tendon reattachment to the footprint was observed, without disruption of the tendon fibers by the needle during implantation of the microfibers.⁴
Clinical Data
Currently, two prospective clinical studies investigating the performance and safety of the FiberLocker® System are ongoing.