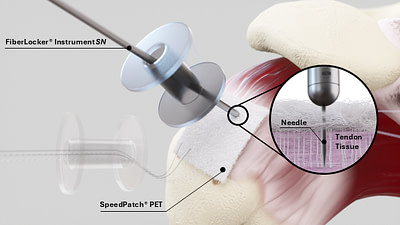
FiberLocker® System
Mechanical Rotator Cuff Augmentation
Clinical Challenge and Proposed Solution
After a tear, the rotator cuff tendon is typically repaired using sutures and bone anchors. Unfortunately, due to its compromised structural integrity, the tendon often cannot withstand suture fixation, leading to a high rate of failure at the suture-tendon interface, commonly referred to as ‘cheesewiring’.1 As a result, postoperative retear rates have been reported to range from 24% to 94%.2,3 The FiberLocker® System is used to mechanically augment the rotator cuff, providing immediate structural integrity to the repaired and injured tendon after surgery.
Sources
FiberLocker® System
Mechanical Rotator Cuff Augmentation
Clinical Challenge and Proposed Solution
After a tear, the rotator cuff tendon is typically repaired using sutures and bone anchors. Unfortunately, due to its compromised structural integrity, the tendon often cannot withstand suture fixation, leading to a high rate of failure at the suture-tendon interface, commonly referred to as ‘cheesewiring’.¹ As a result, postoperative retear rates have been reported to range from 25% to 94%.²·³ The FiberLocker® System is used to mechanically augment the rotator cuff, providing immediate structural integrity to the repaired and injured tendon after surgery.
Sources
¹Cummins CA, Murrell GA. Mode of failure for rotator cuff repair with suture anchors identified at revision surgery. J Shoulder Elbow Surg. 2003 Mar-Apr;12(2):128-33. doi: 10.1067/mse.2003.21. PMID: 12700563.
² Kwon J, Kim SH, Lee YH, Kim TI, Oh JH. The Rotator Cuff Healing Index: A New Scoring System to Predict Rotator Cuff Healing After Surgical Repair. Am J Sports Med. 2019 Jan;47(1):173-180. doi: 10.1177/0363546518810763. Epub 2018 Nov 28. PMID: 30485753.
³ Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004 Feb;86(2):219-24. doi: 10.2106/00004623-200402000-00002. PMID: 14960664.
Where to Meet Us
ISCRA (International SCR Association)
February 20-21, 2026 in Sonoma, CA
Day(s)
:
Hour(s)
:
Minute(s)
:
Second(s)
Shoulder 360 Annual Meeting
April 23-25, 2026 in Miami Beach, FL
Day(s)
:
Hour(s)
:
Minute(s)
:
Second(s)
What Experts Say
Our technology is driven by a deep understanding of clinical needs and validated by the surgeons who use it. World-class experts share their experience with the FiberLocker® System and its role in elevating patient outcomes in rotator cuff repair.
“It fills me with pride and joy being able to work with this young and motivated team and I am convinced that the FiberLocker® System has the potential to become a game changer in shoulder surgery.”
Where to Meet Us
ISCRA (International SCR Association)
February 20-21, 2026 in Sonoma, CA
Day(s)
:
Hour(s)
:
Minute(s)
:
Second(s)
Shoulder 360 Annual Meeting
April 23-25, 2026 in Miami Beach, FL
Day(s)
:
Hour(s)
:
Minute(s)
:
Second(s)
News
Discover our LinkedIn feed