FiberLocker® System
The FiberLocker® System is a single-use device intended to be used for reinforcement of the rotator cuff, following or during repair by suture or suture anchors, where weakness exists in the soft tissue.
![]()
FiberLocker® System
The FiberLocker® System is a single-use device intended to be used for reinforcement of the rotator cuff, following or during repair by suture or suture anchors, where weakness exists in the soft tissue.
![]()
Product Overview

SpeedPatch® PET
Implant consisting of non-woven polyethylene terephthalate (PET) fibers.
Size: 30mm x 25mm
FiberLocker® Instrument SN
A single-use surgical instrument for patch implantation, powered by a commercially available shaver unit, is used during arthroscopic procedures.
Product Overview

FiberLocker® Instrument SN
A single-use surgical instrument for patch implantation, powered by a commercially available shaver unit, is used during arthroscopic procedures.
SpeedPatch® PET
Implant consisting of non-woven polyethylene terephthalate (PET) fibers.
Size: 30mm x 25mm

Technology
As demonstrated by ex vivo animal studies, the patented felting technology enables the formation of a strong and interwoven network between the patch and tendon fibers. The reciprocally moving needle of the instrument pushes implant fibers into the underlying tissue. This ensures a patch implantation across the whole-patch tendon interface, leading to improved suture retention properties at time zero.¹
Clinical Application
During the surgical procedure, the graft is secured to the underlying tendon tissue using the FiberLocker® Instrument SN in approximately 90 seconds. Lateral patch-to-bone fixation using suture anchors completes the augmentation.
Technology

As demonstrated by ex vivo animal studies, the patented felting technology enables the formation of a strong and interwoven network between the patch and tendon fibers. The reciprocally moving needle of the instrument pushes pushes implant fibers into the underlying tissue. This ensures a patch implantation across the whole-patch tendon interface, leading to improved suture retention properties at time zero.¹
Supporting Data
Supporting Data
Biomechanical Data
An ex vivo ovine study revealed significantly increased ultimate failure forces at time zero when augmenting a double Mason Allen stitch with the FiberLocker® System compared to a non-augmented control group.¹
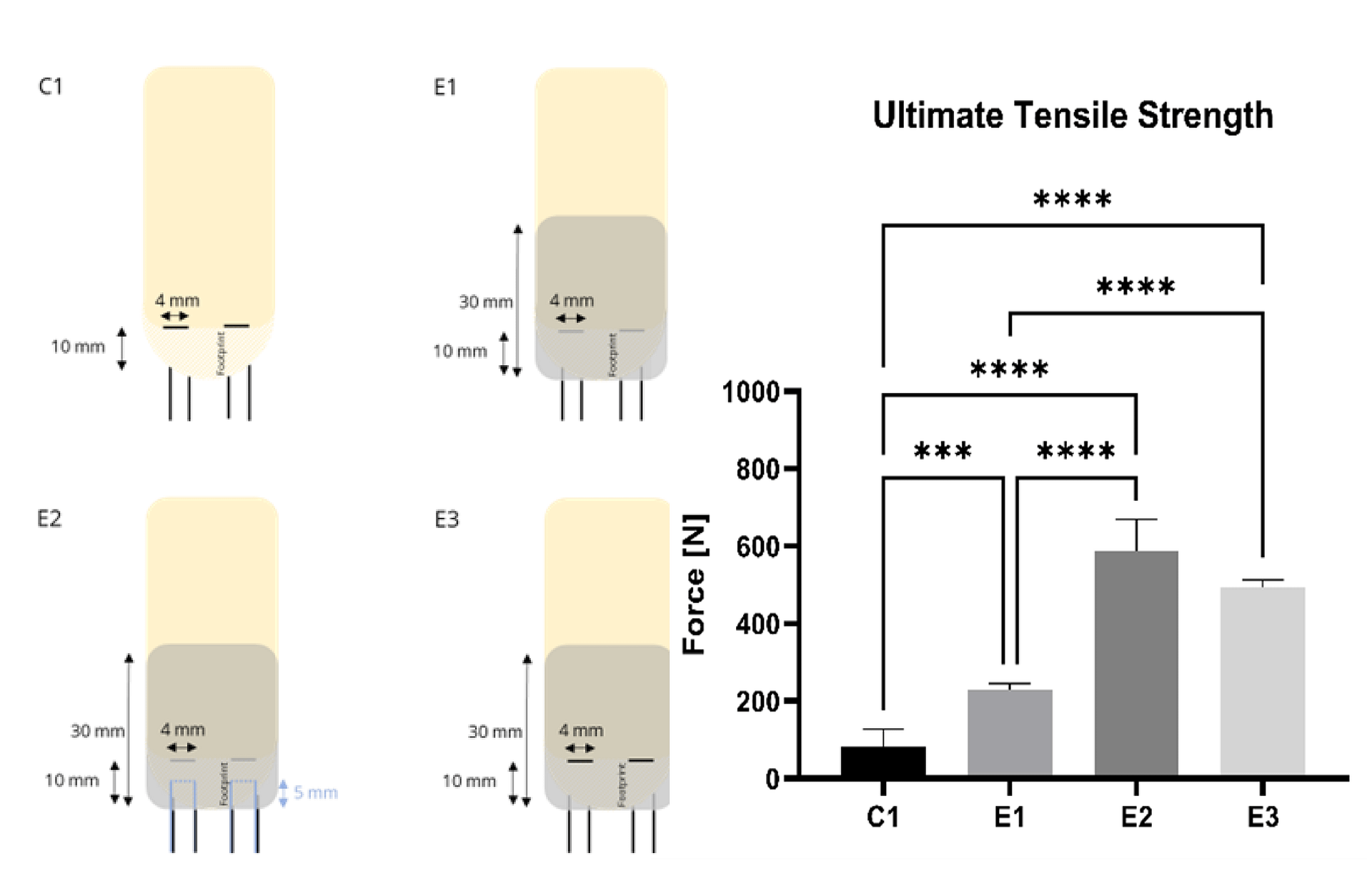
Ex vivo ovine testing of different patch-suture configurations (E1-E3) showed significant enhancement of ultimate tensile strength compared to non-augmented horizontal mattress suture configuration as a control (C1).²
Biological Data
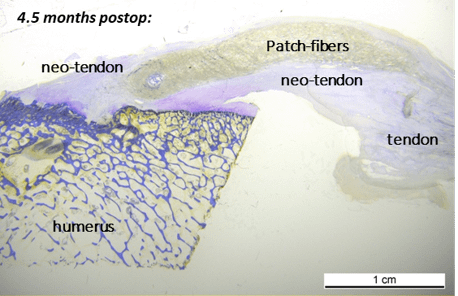
A published ovine in vivo study demonstrated excellent patch integration into the tendon tissue, with a longitudinally aligned ingrown tissue structure. Furthermore, no adverse tissue reactions or capsule formation were observed in any specimens after 13 weeks of implantation.¹
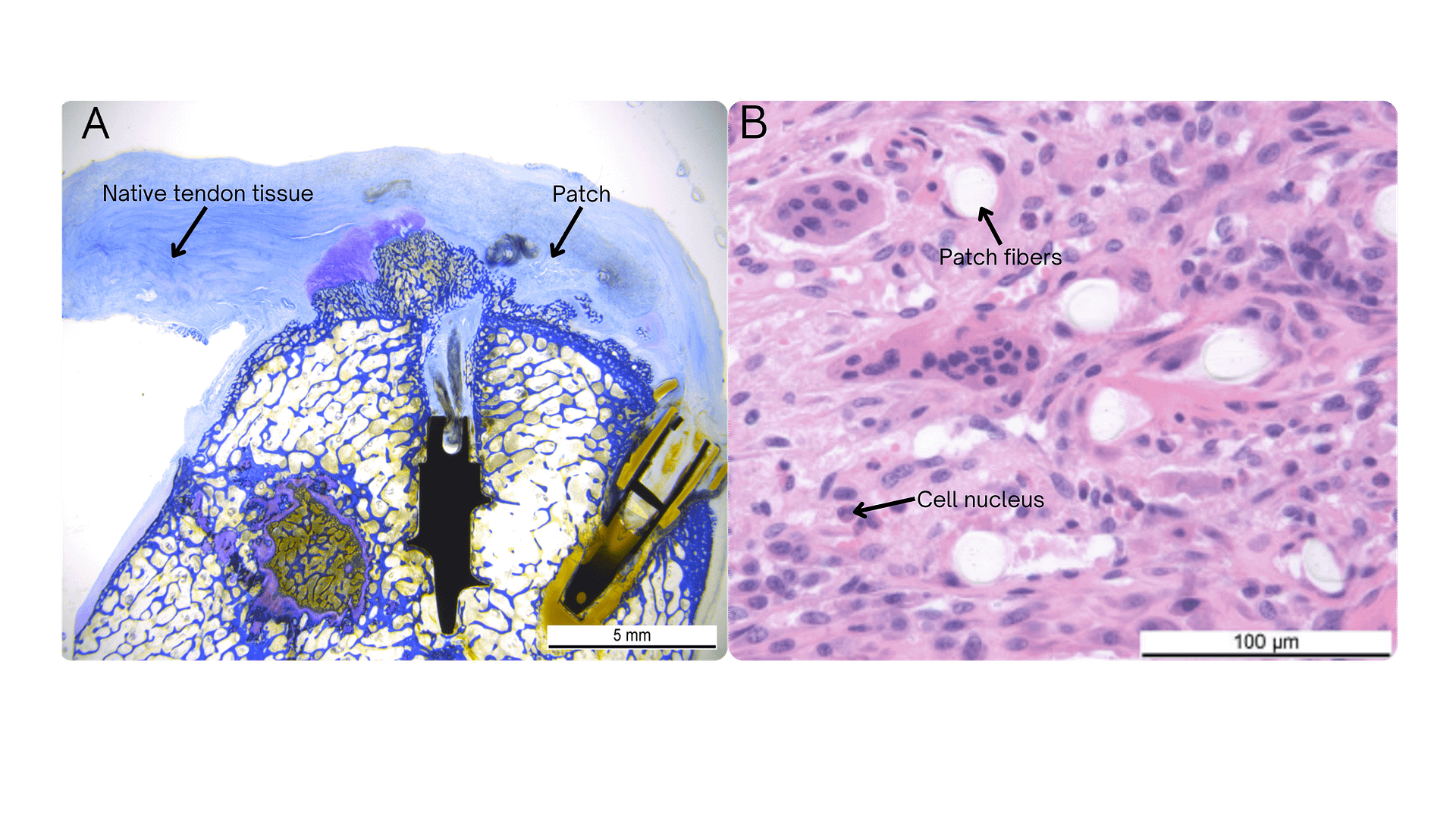
In a second ovine in vivo study, performed according to the Good Laboratory Practice (GLP) principles, no adverse systemic or local side effects were observed. Additionally, directional collagen alignment was shown above the patch (A), and histological examination revealed cell ingrowth within the porous structure of the patch (B).³
Pre-Clinical Cadaver Data
The FiberLocker® System has been tested by over 40 surgeons worldwide during cadaver labs, receiving highly positive feedback and resonance.
Further comparison can be reviewed in publication.
Pre-clinical results may not fully predict outcomes in humans.
² Data on File, ex vivo ovine study using infraspinatus tendon, n = 5/group
³ Data on File, in vivo ovine study using infraspinatus tendon, n = 6/group, 6- and 12-week time point
Interested in using the FiberLocker® System? Contact us for product inquiries.
The FiberLocker® System is currently only commercially available in the U.S.
Biomechanical Data
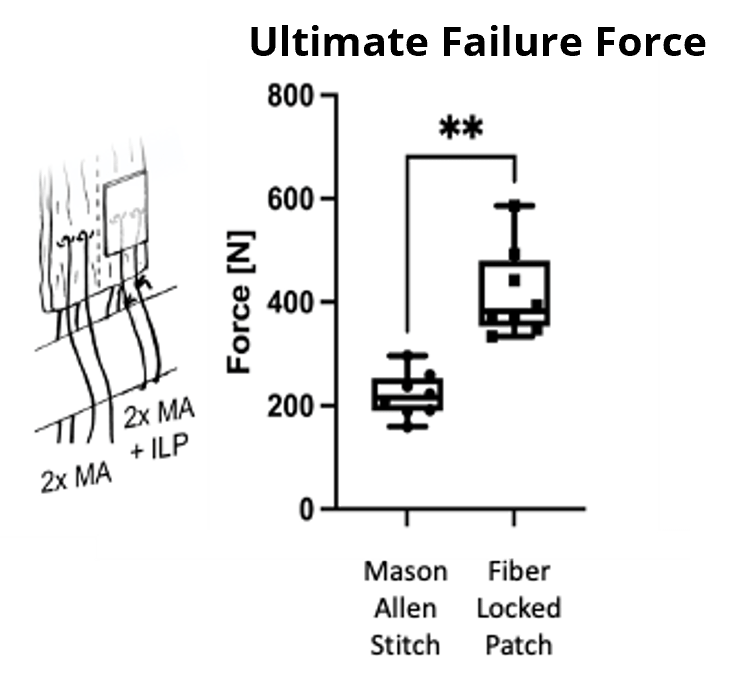
An ex vivo ovine study revealed significantly increased ultimate failure forces at time zero when augmenting a double Mason Allen stitch with the FiberLocker® System compared to a non-augmented control group.¹
Ex vivo ovine testing of different patch-suture configurations (E1-E3) showed significant enhancement of ultimate tensile strength compared to non-augmented horizontal mattress suture configuration as a control (C1).²
Biological Data
A published ovine in vivo study demonstrated excellent patch integration into the tendon tissue, with a longitudinally aligned ingrown tissue structure. Furthermore, no adverse tissue reactions or capsule formation were observed in any specimens after 13 weeks of implantation.¹
In a second ovine in vivo study, performed according to the Good Laboratory Practice (GLP) principles, no adverse systemic or local side effects were observed. Additionally, directional collagen alignment was shown above the patch (A), and histological examination revealed cell ingrowth within the porous structure of the patch (B).³
² Data on File, ex vivo ovine study using infraspinatus tendon, n = 5/group.
³ Data on File, in vivo ovine study using infraspinatus tendon, n = 6/group, 6- and 12-week time point.
Interested in using the FiberLocker® System? Contact us for product inquiries.
The FiberLocker® System is currently only commercially available in the U.S.
Past Development Projects
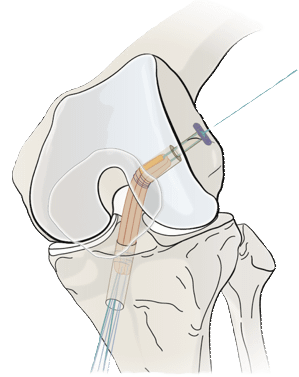
BTB-Converter™
Knee ligament reconstruction.
PRODUCT OVERVIEW
Eight years of advanced biomaterial research and translational development at the University and ETH Zürich, in collaboration with leading Swiss surgeons, has led to ZuriMED’s Bone-Tendon-Bone (BTB)-Converter™ for knee ligament reconstruction.
This technology was developed by ZuriMED in the past and has now been out-licensed.
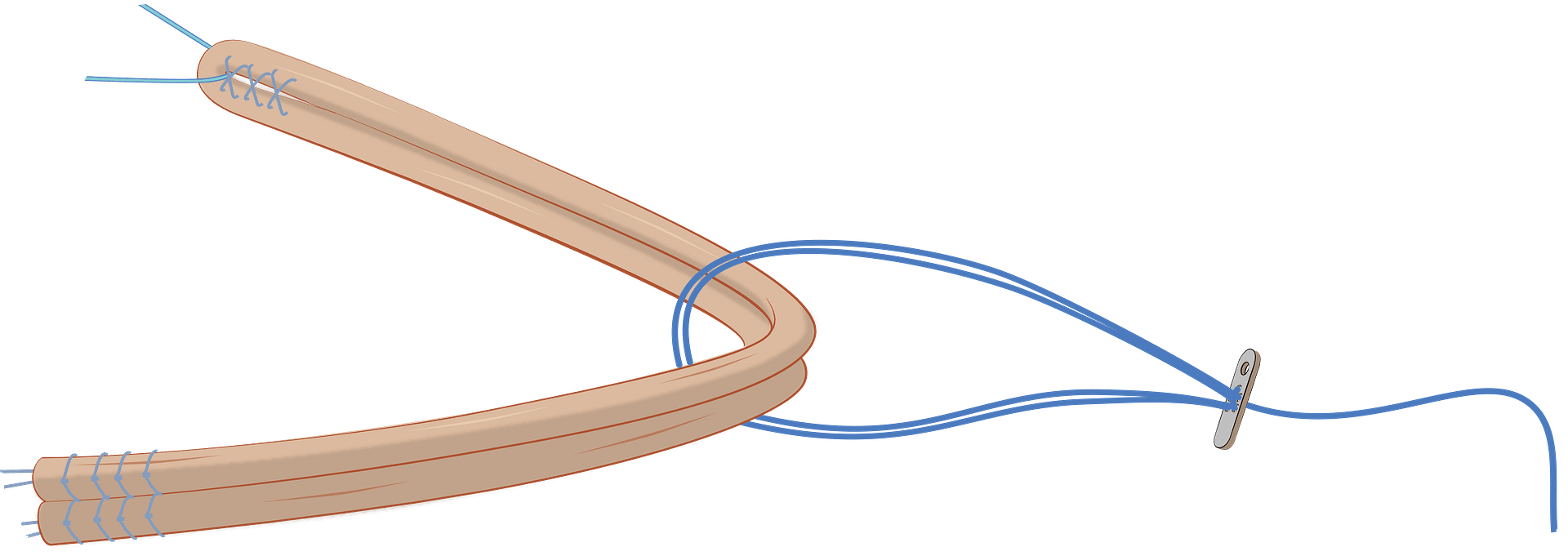
VariLoop™
Superior ligament fixation.
PRODUCT OVERVIEW
The single-strand VariLoop™ enables superior ligament fixation. It is simple, easy to use, self-flipping, and truly knotless. The technology’s high reliability and strength have been proven under all testing conditions. This implant can be used in various configurations for many indications where tension or compression is critical for healing.
This technology was developed by ZuriMED in the past and has now been out-licensed.
ZuriMED is committed to continuously expanding its product portfolio by developing medical devices and technologies that enhance the healing of soft tissue pathologies in patients. Stay tuned for future breakthrough innovations!